High pressure synthesis of phosphine from the elements and the discovery of the missing (PH3)2H2 tile
Matteo Ceppatelli (*,a,b), Demetrio Scelta (a,b), Manuel Serrano-Ruiz (b), Kamil Dziubek (a,b), Gaston Garbarino (c), Jeroen Jacobs J. (c), Mohamed Mezouar (c), Roberto Bini (a,b,d), Maurizio Peruzzini (b)
(a) LENS, European Laboratory for Non-linear Spectroscopy, Via N. Carrara 1, I-50019 Sesto Fiorentino, Firenze, Italy
(b) ICCOM-CNR, Institute of Chemistry of OrganoMetallic Compounds, National Research Council of Italy, Via Madonna del Piano 10, I-50019 Sesto Fiorentino, Firenze, Italy
(c) ESRF, European Synchrotron Radiation Facility, 71 Avenue des Martyrs, 38000 Grenoble, France
(d) Dipartimento di Chimica “Ugo Schiff ” dell’Università degli Studi di Firenze, Via della Lastruccia 3, I-50019 Sesto Fiorentino, Firenze, Italy
(*) Corresponding author: Matteo Ceppatelli (matteo.ceppatelli@iccom.cnr.it and ceppa@lens.unifi.it)
The periodic table of the elements by Mendeleev represents the foundation of our knowledge about the behavior of chemical elements and has recently celebrated its 150th anniversary. During the last few decades, high pressure science has provided experimental evidences of phenomena apparently contrasting our chemical knowledge [1-3]. Nevertheless, before reaching such extreme high density conditions, in which the electronic structure of the atoms is dramatically perturbed, the periodic table as we know it still rules and high pressure has been shown to complement and strengthen its validity.
For example we know that, whereas at ambient pressure the behavior of the first elements belonging to group 14, 15 and 16 (C, N and O) significantly differs with respect to that of the elements in the following period of the same group (Si, P and S), at high pressure these differences tend to fade away, highlighting similarities within the group [4-10].
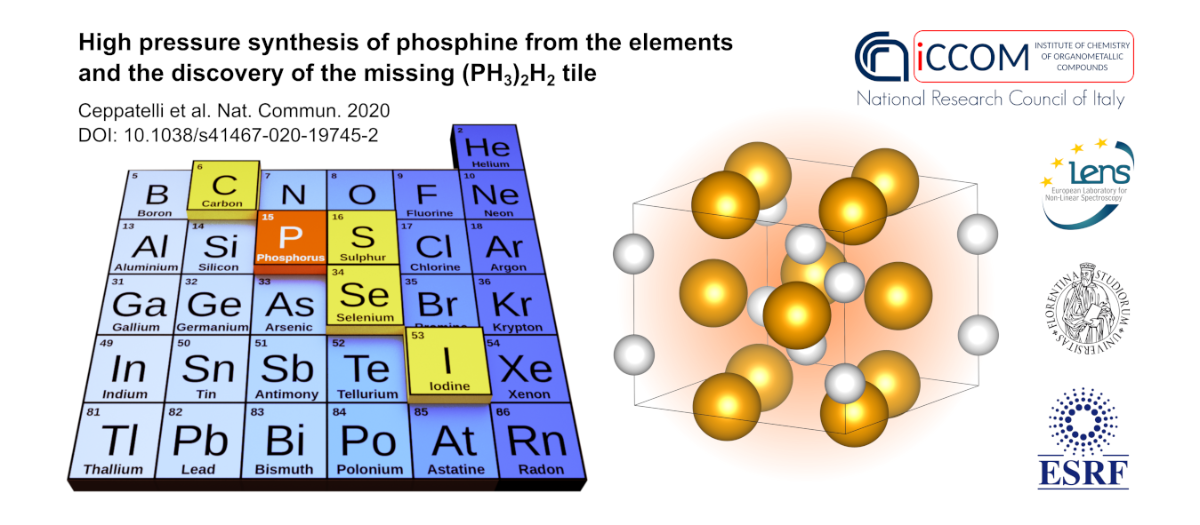
As group 15 is concerned, the catalytic synthesis of ammonia (NH3) from nitrogen (N2) and hydrogen (H2), representing the core reaction of the famous Haber-Bosch process, is certainly one of the most important chemical reactions for humanity, allowing to produce fertilizers from atmospheric nitrogen. At the moment, despite being searched for a long time, the analogous reaction leading to the formation of phosphine (PH3) from phosphorus (the heavier element of group 15 with respect to N) and hydrogen has not yet been reported. In a similar way, a whole class of compounds, called van der Waals (vdW) compounds, made by H2 molecules and by the molecular hydride of a non-metallic element belonging to group 14 to 17 is known at high pressure, with the exception of group 15.
Using synchrotron X-ray diffraction (XRD) at the high pressure beamline ID27 of the European Synchrotron Radiation Facility (ESRF, Grenoble, France) and infrared and Raman spectroscopy at the European Laboratory for Non-Linear Spectroscopy (LENS, Sesto Fiorentino, Italy), researchers of the Institute of Chemistry of OrganoMetallic Compounds of the National Research Council of Italy (ICCOM-CNR), of LENS, of the Department of Chemistry “Ugo Schiff” of the University of Florence and of ESRF demonstrated for the first time that black phosphorus (bP) and molecular hydrogen (H2) directly react without any catalyst to form phosphine under high pressure and high temperature conditions, identifying a new inorganic route to the synthesis of PH3 from the elements analogous the synthesis of NH3 from N2 and H2. To perform this experiment, a small crystal of high purity bP, synthesized at ICCOM-CNR, was placed in a pressure generating device called Diamond Anvil Cell (DAC), which was then gas-loaded with H2. The sample was compressed to 1.2 GPa (~12000 times the atmospheric pressure) and heated by means of infrared laser irradiation to temperature as high as 1000 K (727 °C), up to the occurrence of chemical reactivity.
Moreover, the authors of this study discovered that at higher pressure (3.5-4.1 GPa) and room temperature PH3 and H2 form a crystalline van der Waals compound (PH3)2H2, which was never before observed for any of group 15 elements, thus representing the so far missing tile for pnictogens in the puzzle of the periodic table concerning the ability of non-metallic elements to form this kind of compounds.
The results of this study have multiple relevant implications encompassing fundamental chemistry of the elements and bond theory under high pressure conditions, the synthesis of energetic high-H2-content and superconducting materials, and astrochemistry issues related to the presence of PH3 and H2 in extraterrestrial environment of giant planets such as Jupiter, Saturn and their moons, and to the recent claim of the presence of life on Venus, based on the detection of apparently anomalous high level of PH3 in the cloud decks of the planet.
Nature Communications 2020, 11, 6125; doi: 10.1038/s41467-020-19745-2.
https://www.nature.com/articles/s41467-020-19745-2
References
[1] Paul Loubeyre, Florent Occelli, Paul Dumas “Synchrotron infrared spectroscopic evidence of the probable transition to metal hydrogen” Nature 2020, 577, 631.
[2] Yanming Ma, Mikhail Eremets, Artem R. Oganov, Yu Xie, Ivan Trojan, Sergey Medvedev, Andriy O. Lyakhov, Mario Valle, Vitali Prakapenka “Transparent dense sodium” Nature 2009, 45, 182.
[3] Xiao Dong, Artem R. Oganov, Alexander F. Goncharov, Elissaios Stavrou, Sergey Lobanov, Gabriele Saleh, Guang-Rui Qian, Qiang Zhu, Carlo Gatti, Volker L. Deringer, Richard Dronskowski, Xiang-Feng Zhou, Vitali B. Prakapenka, Zuzana Konôpková, Ivan A. Popov, Alexander I. Boldyrev and Hui-Tian Wang “A stable compound of helium and sodium at high pressure” Nat. Chem. 2017, 9, 440.
[4] Mikhail I. Eremets, Alexander G. Gavriliuk, Ivan A. Trojan, Dymitro A. Dzivenko, Reinhard Boehler, “Single-bonded cubic form of nitrogen” Nat. Mater. 2004, 3, 558.
[5] Lars F. Lundegaard, Gunnar Weck, Malcolm I. McMahon, Serge Desgreniers, Paul Loubeyre “Observation of an O8 molecular lattice in the ε phase of solid oxygen” Nature, 2006, 443, 201.
[6] Mario Santoro, Federico A. Gorelli, Roberto Bini, Giancarlo Ruocco, Sandro Scandolo, Wilson A. Crichton “Amorphous silica-like carbon dioxide”, Nature 2006, 441, 857.
[7] Dominique Laniel, Bjoern Winkler, Timofey Fedotenko, Anna Pakhomova, Stella Chariton, Victor Milman, Vitali Prakapenka, Leonid Dubrovinsky, Natalia Dubrovinskaia “High-Pressure Polymeric Nitrogen Allotrope with the Black Phosphorus Structure” Phys. Rev. Lett. 2020, 124, 216001.
[8] Cheng Ji, Adebayo A. Adeleke, Liuxiang Yang, Biao Wan, Huiyang Gou, Yansun Yao, Bing Li, Yue Meng, Jesse S. Smith, Vitali B. Prakapenka, Wenjun Liu, Guoyin Shen, Wendy L. Mao, Ho-kwang Mao
“Nitrogen in black phosphorus structure” Sci. Adv. 2020, 6, eaba9206.
[9] Demetrio Scelta D., Adhara Baldassarre, Manuel Serrano-Ruiz, Kamil Dziubek, Andrew. B. Cairns, Maurizio Peruzzini, Roberto Bini, Matteo Ceppatelli “Interlayer Bond Formation in Black Phosphorus at High Pressure” Angew. Chem. Int. Ed. 14135, 56, 2017.
[10] Demetrio Scelta, Adhara Baldassarre, Manuel Serrano-Ruiz, Kamil Dziubek, Andrew B, Cairns, Maurizio Peruzzini, Roberto Bini, Matteo Ceppatelli “The p-sc structure in phosphorus: bringing order to the high pressure phases of group 15 elements” Chem. Commun. 54, 10554 2018.
